
Application Note
High-throughput IgG quantitation platform for clone screening during drug discovery and development
- Fast, homogeneous, and automation-friendly assay with results for 96 samples in less than 15 minutes
- Low sample volume and limited test sample pre-preparation, allowing analysis of samples in a crude matrix
- Precise measurement of IgG from 2.5 to 2000 mg/L
Cathy Olsen, PhD | Sr. Applications Scientist | Molecular Devices
Hannah Byrne, PhD | Head of Biological Sciences | Valitacell
Introduction
Biologic drugs are the largest and fastest growing segment of the pharmaceutical industry with sales of €500bn and an annual growth of 8%. Every manufacturing process for potential biologics begins with cell line development, whether it’s for clinical trials or a market launch. Monoclonal antibodies (mAb) have established themselves as the leading biopharmaceutical therapeutic modality. The establishment of robust manufacturing platforms is key for antibody drug discovery efforts to seamlessly translate into clinical and commercial successes. The accurate and reliable measurement of mAb (e.g., IgG) titer is essential in the development and subsequent manufacture to ensure optimal cell culture performance for the production of all biologics (Figure 1). The ability to reliably monitor protein titer in real time throughout a bioprocess allows operators to rapidly adjust the process conditions for maximum protein output while minimizing process time. Quick access to titer data also enables earlier decisions regarding preparation of downstream processes, further reducing the production timeline.
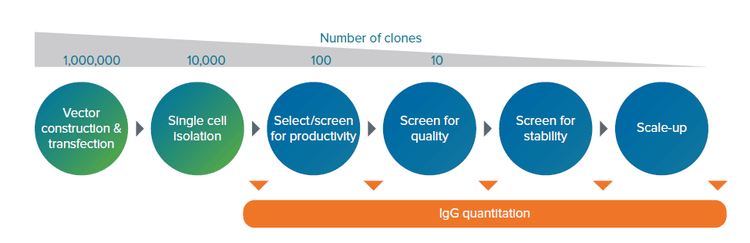
Figure 1. The cell line development process from cell transfection to scale-up. The cell line generation process is highly complex, tedious, and time consuming as clones with high productivity, stable long-term expression and good product quality are rare occurrences. Hence, screening strategies are implemented at all stages of biologic drug production. The slowest step in developing a new mAb with therapeutic potential is clone selection, which is hindered today by legacy screening technologies. Additionally, current workflows are single function, costly, and require specialized training.
Of the various technologies currently employed by the biopharmaceutical industry to quantify mAbs, the gold standard Protein A HPLC, bio-layer interferometry, enzyme-linked immunosorbent assay (ELISA), and immunoturbidimetric assays are common methods. They all have distinct features including cost per test, cost of hardware, and experience of staff required to execute the experiment. Importantly, some of these techniques require various steps to prepare the samples for analysis, such as centrifugation or dilution to remove whole cells, cellular debris, and contaminants. Despite their widespread adoption in industry, the high cost (Protein A HPLC), sensitivity to cellular contamination leading to variability in results, susceptibility to human error, labor intensive workflow (ELISA), and slow time-to-result (>3 hours in some cases) remain as big hurdles for users looking to adopt Protein A HPLC and ELISA throughout their bioprocessing workflows for the quantitation of IgG.
Here we provide an overview of a fully optimized rapid, robust, and accurate IgG titer platform combining ValitaCell’s ValitaTiter IgG quantitation assay with a suite of Molecular Devices fluorescence polarization (FP) configured microplate readers. The ValitaTiter assay range measures IgG concentrations from 2.5 to 100 mg/L or 100 mg/L to 2000 mg/L, with a simple add-and-read protocol. ValitaTiter plates come precoated with a fluorescently-labeled, target-specific probe that the user reconstitutes prior to IgG test sample addition. The assay is performed in less than 15 minutes and can be incorporated into the bioprocess workflow in a 96- or 384-well plate format. The assays are high throughput and can be fully automated. Analysis can be carried out in crude cell culture media containing up to 10 x 106 cells/mL with a low sample volume and limited test sample pre-preparation. Assay detection can be performed using fluorescence polarization on Molecular Devices microplate readers: SpectraMax® iD5, i3x, Paradigm®, and M5 Multi-Mode Microplate Readers. (The i3x and Paradigm readers require the Fluorescence Polarization Detection Cartridge.)
The SpectraMax Multi-Mode Microplate Readers provide excellent flexibility, and most include absorbance, fluorescence, and luminescence with configurable options for fluorescence polarization (FP), time-resolved fluorescence (TRF), and FRET. Upgradeable modules are also available including western blot, cell imaging, and injectors for fast kinetics.
10 x 10
6
million cells/mL
Table 1. Overview of key features of Valita®TITER versus competitors.
Assay principle
ValitaTiter and ValitaTiter Plus are rapid, high-throughput assays quantifying IgG-Fc interactions with a fluorescently labeled derivative of protein G using FP for detection. FP effectively analyzes changes in the size of molecules (Figure 2). “Fixed” fluorophores are excited by polarized light and preferentially emit light in the same plane of polarization. The rotation of the molecules between absorption and emission of the photon results in “twisting” the polarization of the light. Small molecules tumble faster in solution than larger molecules. Hence, the change in molecule size upon the binding of a fluorescently labeled Fc-specific probe can be detected using the degree of light depolarization. When the fluorescently labeled IgG-binding peptide is unbound, it tumbles rapidly, depolarizing the light more than when bound to an IgG (which is ~20 times larger). The detection of FP involves excitation of the solution with plane polarized light and subsequent measurement of emitted light intensity in both the parallel (polarized portion) and perpendicular (depolarized portion) planes to the exciting light. The FP is expressed as a normalized difference of the two intensities, typically expressed in millipolarization units (mP).
Materials
- ValitaCell ValitaTiter Kit (cat. #VAL003)
- ValitaCell ValitaTiter Plus Kit (cat. #VAL004)
- Sigma IgG standard (Sigma cat. #I2511)
- XP Media™ CHO Growth A (Molecular Devices cat. #K8860), supplemented with 4 mM L-glutamine
- SpectraMax iD5 Multi-Mode Microplate Reader
- Set of 2 Fluorescence Polarization Filters 485 nm BW 25 nm Polarized Vertical & Horizontal (Molecular Devices cat. #6590-0136)
- Set of 2 Fluorescence Polarization Filters 535 nm BW 25 nm Polarized Vertical & Horizontal (Molecular Devices cat. #6590-0137)
- SpectraMax i3x Multi-Mode Microplate Reader
- Fluorescence Polarization (FP-FLUO) Detection Cartridge (Molecular Devices cat. #0200-7009
- SpectraMax Paradigm Multi-Mode Microplate Reader
- Fluorescence Polarization (FP-FLUO) Detection Cartridge (Molecular Devices cat. #0200-7009)
- SpectraMax M5 Multi-Mode Microplate Reader
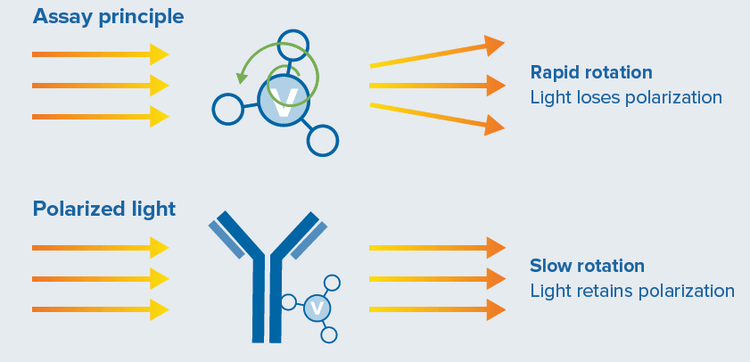
Figure 2. The assay applies fluorescence polarization to quantify Fc-containing IgG. Small, unbound molecules rotate rapidly in solution (top), while large, bound molecules rotate slowly (bottom).
Method
- A serial dilution of IgG standards was performed, using XP Media/L-glutamine as the diluent, to concentrations from 2.5–100mg/L (ValitaTiter) or 100–2000mg/L (ValitaTiter Plus).
- 60 µL of medium was pipetted into each well of the ValitaTiter or ValitaTiter Plus plate to reconstitute the probe.
- 60 µL of prepared standards were then added to the appropriate wells.
- Well contents were mixed by gently pipetting up and down three times (see Figure 3 for assay workflow overview).
- Assay plates were incubated in the dark for five minutes (ValitaTiter) or 15 minutes (ValitaTiter Plus) at room temperature prior to measurement on a suite of Molecular Devices microplate readers, using the identified fully optimized settings outlined in Table 2 (ValitaTiter) and Table 3 (ValitaTiter Plus).
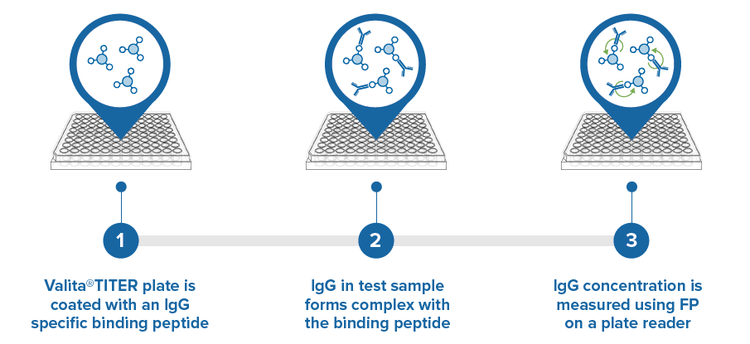
Figure 3. Each well of the assay plate is precoated with a fluorescently labeled Fc-specific probe (1). An IgG sample binds to the probe (2). Binding is measured via fluorescence polarization (3).
Table 2. Optimal instrument settings for Valita®TITER assay Fluorescence Polarization measurement on Molecular Devices microplate readers. SpectraMax i3x and Paradigm readers require the FP-FLUO detection cartridge. Settings not required by a reader are indicated by ‘---’.
Table 3. Optimal instrument settings for Valita®TITER Plus assay Fluorescence Polarization measurement on Molecular Devices microplate readers. SpectraMax i3x and Paradigm readers require the FP-FLUO detection cartridge. Settings not required by a reader are indicated by ‘---’.
Results
An investigation was carried out in order to identify the optimal parameters for using ValitaCell’s ValitaTiter assays on Molecular Devices suite of multi-mode microplate readers in order to provide a cost-effective, high-throughput IgG quantitation platform for use in high-throughput drug discovery and development. IgG standard curves were prepared and analyzed using a simple add-and-read method, with no sample or plate pre-preparation or wash steps required, and an easy workflow.
Superior results were obtained using a 485 nm FP filter set for excitation, and a 535 nm FP filter set for emission, using the SpectraMax iD5 reader. IgG Standards from 2.5mg/L to 100mg/L (ValitaTiter) or 100mg/L to 2000mg/L (ValitaTiter Plus) were detected with a high degree of linearity (R2=0.99) across the entire range (Figure 4). Comparable data for ValitaTiter (Table 4) and ValitaTiter Plus assay (Table 5) were obtained with the SpectraMax i3x, Paradigm, and M5 readers. A preconfigured protocol in SoftMax® Pro Software automated the mP calculations and curve plotting.
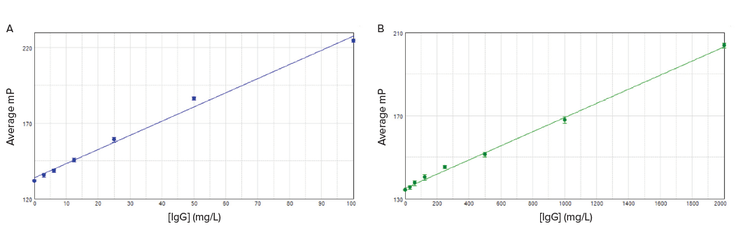
Figure 4. Standard curves for the ValitaTiter (A, r2 = 0.993) and ValitaTiter Plus (B, r2 = 0.998) assays. Curves were plotted using a linear curve fit in SoftMax Pro Software.
Table 4. Standard delta mP, average standard deviation (StDev) and %CV (n = 4) for Valita®TITER standards read on Molecular Devices readers.
Table 5. Standard delta mP, average standard deviation (StDev) and %CV (n = 4) for Valita®TITER Plus standards read on Molecular Devices readers.
Conclusion
The accurate and reliable measurement of mAb IgG titer is essential in the development and subsequent manufacture to ensure optimal cell culture performance for the production of all biologics. An assay that enables accurate results with a minimal investment of time and resources is critical to success. Here, we successfully demonstrate that ValitaTiter assays combined with Molecular Devices microplate readers enable quantitation of IgG across a wide functional range.
The ValitaTiter assay is a homogeneous, high-throughput method for precise and rapid quantitation of IgG in crude samples, without the requirement of sample preparation or purification steps. This 96-well assay has been fully validated on the SpectraMax iD5 reader and other Molecular Devices microplate readers with FP detection to ensure reliable results. SoftMax Pro Software minimizes setup time for detection and automates standard curve fitting and sample quantitation.