
Application Note
Automated dispensing, monitoring, and assay development of hydrogel-based cellular models
- Integrate workflows for the development, maintenance, and imaging of 3D cellular models
- User-friendly—and cGMP-certified—interface allows for easy integration and operation between the BioAssemblyBot® 400 and ImageXpress® portfolio of imagers
- Temperature-independent, xeno-free matrix for 3D cellular models for high-throughput assays
- Improve your high-throughput, high-content imaging assays for 3D cellular models with automated BAB400 and ImageXpress® Micro Confocal High-Content Imaging System (IXM-C)
Prathyushakrishna Macha, PhD | Research Scientist | Molecular Devices
Introduction
3D cellular models that more accurately represent various microenvironments are incredibly important for accurate drug screening and disease modeling. These can be used as in vitro and in vivo models to conduct high-throughput, cost-effective assays that are vital to discovering biologically relevant therapies. To adopt 3D models more widely in drug discovery research and screening—and to make the labor-intensive processes simpler—there is an increasing need for high-throughput screening and automated processing to significantly reduce cost and time to market.
The BioAssemblyBot 400 (BAB400) by Advanced Solutions is a cGMP-certified multitool that can be used for setting up complex biological models. A multipurpose platform that can be used to build models, its pneumatic dispensing and 3D printing capabilities enable it to build and handle different 3D models or organoids without damage. Its liquid handling tool effectively dispenses the prescribed number of cells in a hydrogel, Matrigel®, or other suitable extracellular matrices into multiwell plates. From there, it can add or exchange media, transport plates, and be incorporated into a smart, integrated, automated workcell to perform basic, pharmacological, and biomedical workflows.
In this work we used VitroGel® matrix for printing 3D cell structures with cells in 96-well format. VitroGel from The Well Bioscience is a tunable, xeno-free (animal originfree) bio-functional hydrogel system that allows maximum flexibility to manipulate the 3D cell culture environment. It is temperature-independent and an excellent alternative to animal-based extracellular matrices (ECM), like Matrigel, and can support a wide range of cell types and end structure requirements. The unique shear-thinning and rapid recovery rheological properties of VitroGel enable excellent cell distribution after dispensing and make it extremely easy to use with an automated liquid handling system.
In this work we used patient-derived triple-negative breast cancer cells (citation) mixed with VitroGel matrix to create 3D cultures by bio-printing in 96-well format that can be used for observation of cell growth as 3D tumoroids evaluation of effects of drugs.
We present a workflow for 3D cellular models using VitroGel in an integrated system to dispense, monitor, perform drug screening assays, and image and analyze data. This system includes the BAB400, and the ImageXpress Micro Confocal system (Figure 1).
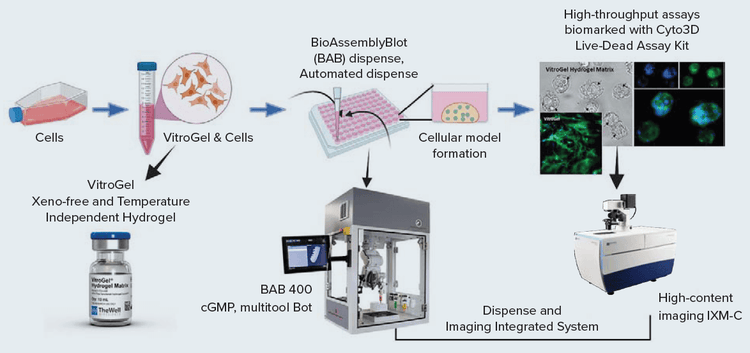
Figure 1. Schematic representation of Integrated system workflow [Automated dispense by BAB and Imaging by IXM-C]
Methods
VitroGel and cell suspension for spheroids
4IC cells1 were cultured with advanced DMEM supplemented with glucose, NEAA, 2mM glutamine, and insulin 120μg/L, 10% FBS (Gibco 12491-015). For assays, spheroids were cultured with DMEM + 10% dialyzed serum (2mM glutamine, 5mM glucose, without phenol red).
The patient-derived triple-negative breast cancer cells were harvested using trypsin and suspended in a medium with 30% FBS at a concentration of 5 X 105 cells/mL. The 3D cell culture was performed in VitroGel Hydrogel Matrix (SKU: VHM01) with the following steps:
- Bring the VitroGel Hydrogel Matrix to room temperature or warm up to 37°C.
- Mix the hydrogel solution and the cell suspension at a 2:1 (v/v) ratio.
- Add 10 μL of the hydrogel-cell mixture to each well of a 96-well plate (Non-Tissue Culture Treated)
- Wait 10–15 minutes at room temperature for hydrogel formation. Do not disturb the hydrogel by tilting or shaking the well plate during the formation process.
- Carefully add 90 μL cell culture medium on top of the hydrogel.
- Place the well plate in an incubator and change the cover medium every other day.
BioAssemblyBot 400 for dispensing (pipette tool), media exchange, drug screening, and live and dead assays
The BAB400 provides a sterile environment with a HEPA air flow system. Spray and wipe the surfaces except for tools and any electronics and turn on the HEPA filter functionality at least an hour (overnight preferred) prior to use. Using BAB400’s human machine interface (HMI) and a joystick controller, different paths can be designed for various steps. The paths are set for each workflow desired using the console to record the coordinates of the reservoir, plate, tubes, trash, etc. The media and cell suspension in the VitroGel are prepared beforehand in the BAB400 and transferred to the reservoir.
Gripper (PnP tool) Sequence can be used to de-lid and lid the plates, and to move the plates to the ImageXpress Micro Confocal system for imaging once dispensing is completed using BAB400. BAB400 has the option of running multi-step processes without human interaction. Experiments can be turned into automated workflows by stitching together custom experimental protocols to build 3D cellular models. BioApps™ Maker is an easy-to-make and easy-to-automate solution that was used to automate a sequence of steps for independent dispensing, maintenance, and imaging of the 3D models.
For example, the hydrogel dispenses sequence steps involved are laid out:
Get tip → Pipette tool moves to the hydrogel reservoir → down (into the reservoir) → Mix Y number of times (aspirate followed by dispense at defined speed) → Move to the plate (left/right/directions – then well center) → Dispense (define volume and dispense speed) → Trash the tips (into a biohazard bag in BAB400). A similar path could be designed for media aspiration and dispense – the tip goes to the edge of the wells to keep the VitroGel domes intact. Media with select drugs and Live/dead stain addition can also be used for further treatments and imaging assays of the spheroids. Optional step: Precooling the temperaturecontrolled stage of BAB400 to 10°C for faster gelation of dispensed VitroGel droplets in the 96-well.
ImageXpress Micro Confocal Imaging and analysis
After the BAB400 moves the microwell plates to the ImageXpress Micro Confocal imager (Molecular Devices), transmitted light (TL) or fluorescent images were acquired using MetaXpress® High-Content Image Acquisition & Analysis Software. Z-stack images for the spheroids were acquired with the 4X or 10X objectives using confocal mode. MetaXpress Image Analysis Software was used for all analyses. BioApps™ can pull up and execute the right preselected imaging acquisition and analysis files for a particular sequence or experiment.
Results
Multiple 96-well plates were plated with 4IC breast cancer cells in VitroGel domes were set up by both the BAB400 and manual dispenses. These were observed on day 3 using the Cyto3D® Live-Dead Assay Kit (TheWell Bioscience, SKU: BM01) stain for live and dead counts, and when analyzed by using imaging method as described above. Live-dead analysis was used for counting live and dead cells. Apparently, there wasn’t a significant difference in cell viability between cells plated manually or automatically. The BAB400 workflow had an average viability of 93%, whereas the manually dispensed well plates had a viability of 92.4% (Figure 2). Therefore, automated cell printing did not impact cell viability.
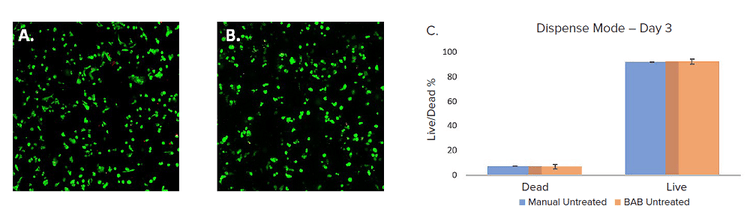
Figure 2. A. BAB B. Manual, dispensed VitroGel and triple-negative breast cancer cells (4IC) stained with Cyto3D ( Green – live, Red – dead ) and imaged on Day 3 using IXM-C. The graphical representation of data analyzed of the same (C.) using MetaXpress software.
To test the workflow for drug treatment, the drugs Trametinib and Idarubicin were added to the multiwell plates on day 3 to make up a final concentration of 4μM and their effects were evaluated on day 5 after 48h of treatment. We observed a significant decrease in cell viability in both drug treatments when compared to the control (Figure 3). Cell viability was determined by imaging methods after staining with viability dyes as described above. The dead cells percentage in idarubicin 4μM was the highest with 97% of cells in spheroids dead, whereas in the case of trametinib it was 66.4% and the control was 7.5% (Figure 3).
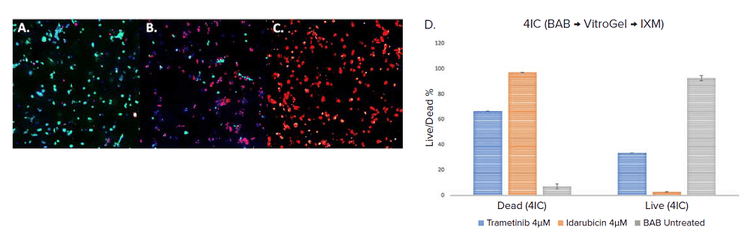
Figure 3. Cells were dispensed with BAB (VitroGel and triple-negative breast cancer cells (4IC) ). Control (A.) and treated samples were stained with Cyto3D ( Green – live, Red – dead ) and DAPI (Blue – nuclei), treated – 48 h treatment with Trametinib (B.) and Idarubicin (C.) at 4μM concentration. These were imaged on Day 5 using IXM-C. The graphical representation of data analyzed of the same (D.) using MetaXpress software.
Conclusion
The 3D cellular model workflow can be automated by integrating the BAB400 and ImageXpress Micro Confocal imager with an easy-to-handle and tunable ECM matrix (VitroGel) for 2D/3D cell culture, maintenance, and differentiation of 3D cellular models that can be used for compound screening and a variety of assays. This will effectively contribute to the ongoing basic, pharmacological, and biomedical research and product development by saving time and cost and reducing repetitive steps.
Acknowledgement
Grateful for the patient-derived cell lines from Dr. Matthew E. Burow; Associate Professor of Medicine and Tulane Cancer Center Program Member
Reference
- Cromwell EF, Sirenko O, Nikolov E, Hammer M, Brock CK, Matossian MD, Alzoubi MS, Collins-Burow BM, Burow ME. Multifunctional profiling of triple-negative breast cancer patient-derived tumoroids for disease modeling, SLAS Discovery 2022; 27(3): 191–200.