
Application Note
Confident Identification of Monoclonal CHO-S Cells Grown in Semi-Solid Media using the CloneSelect Imager
- Quickly identify monoclonal cell lines with ultra-fast imaging speed
- Track the growth of immobilized single cells with confidence
- Image single cells in semi-solid or liquid media with great experimental flexibility
Introduction
Regulatory criteria to obtain approval of biological drugs are constantly evolving. One of the regulatory metrics for biological cell lines, the digital documentation of monoclonality, has driven the need for more robust technologies and methodologies in bioprocessing. Many researchers now routinely use imaging systems, such as the CloneSelect™ Imager, to verify monoclonality and monitor cell growth in liquid cell culture media. However, the CloneSelect Imager also has the flexibility to image cells grown in semi-solid media which remain stationary, allowing researchers to confidently know that they are imaging the same cells over time.
Traditionally, limiting dilution has been used to clone cells. However, this method uses liquid media which can make cell-growth tracking challenging as normal cell culture plate handling can cause suspension cells to move within the wells (Figure 1). This cell movement decreases confidence that the same cell is being imaged. With limiting dilution it is also possible that more than one cell is seeded into a well which may make it necessary to perform additional subcloning to obtain a high probability of monoclonality.
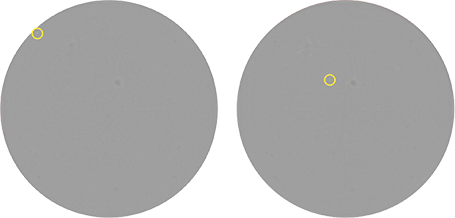
Figure 1: Images of the same well from a limiting dilution seeding of CHO-S cells in a 96-well plate. The plates were imaged 8 hours apart on the same day on the CloneSelect Imager. With just routine plate handling, the cell has already shifted between scans. The yellow circle shows the location of the cell.
Growing cells in semi-solid media provides researchers with a faster and easier solution for cloning and tracking growth of cells. Cells grown in semi-solid media are immobilized, preventing them from moving during routine handling while allowing them to grow and form distinct clonal colonies. This makes it possible to seed many cells in the same well and still be able to clearly view single cell outgrowth by imaging the cells over time, providing both digital verification of monoclonality and growth tracking.
The CloneSelect Imager provides researchers with a turnkey solution to image cells in semi-solid media. The CloneSelect Imager is a non-invasive and label-free imaging system that allows for objective, quantitative assessment of cell growth and monoclonality verification which ensures that only stable clonal cell lines are selected for downstream studies. In this application note, we experimentally demonstrate these capabilities on the CloneSelect Imager to image suspension-adapted CHO-S cells grown in CloneMedia™ CHO Growth A, a semi-solid media specially formulated to promote the growth of various CHO host cell lines.
Workflow procedure
CHO-S cell culturing and imaging workflow is shown in Figure 2.

Figure 2: Overview of tracking single cell outgrowth in CloneMedia CHO Growth A media. The XP Media CHO Growth A and CloneMedia CHO Growth A enable a robust method for culturing, cloning and screening CHO cells. Images captured by the CloneSelect Imager allow for monoclonality verification and cell-growth tracking.
CHO-S cell culturing
The CHO-S cells were initially cultured in XP Media™ CHO Growth A liquid media (catalog# K8860) supplemented with 4 mM L-glutamine and incubated at 37°C. CHO-S cells were then seeded into CloneMedia CHO Growth A semi-solid media (catalog# K8810) at a density of 50,000 and 500 cells/mL. The CHO-S cell and CloneMedia CHO Growth A mixtures were pipetted into 6-well plates (Greiner catalog# 657185) at a volume of 2 mL/well. 10 μM diameter nonsterile beads (Thermo Scientific catalog# G1000B) were also added to the semi-solid mixtures. These beads are not required for the CHO cell semi-solid media workflow. The beads serve as location landmark references for the images.
Well A1 was seeded with 2 mL of 50,000 cells/mL to determine the focal position where the majority of CHO-S cells were located. The remaining wells were seeded with 2 mL of 500 cells/mL. The plates were then centrifuged at 200 x g for 5 min and then incubated at 37°C .
CHO-S cell imaging
Plates were imaged using the CloneSelect Imager. The CHO-S cells were imaged at multiple time points to provide digital proof of monoclonality and to track cell growth. Each plate was imaged on Day 0, 1, 2, 7, and 8. For Day 0, plates were incubated for 4 hours at 37°C prior to imaging. On Day 0, autofocus was used on well A1 to determine the appropriate focal position for all wells in the plate (Figure 3). This focus position was used for subsequent images on the remaining time points.
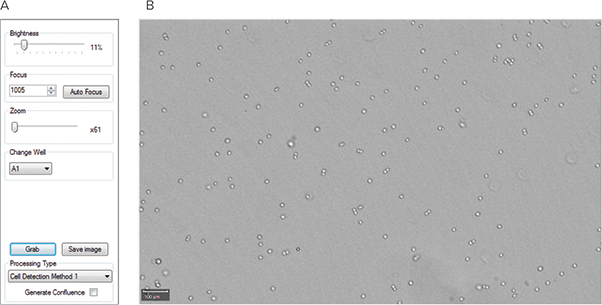
Figure 3: Setting the focus on the CloneSelect Imager. (A) CloneSelect Imager focus settings used to image the CHO-S cells in 6-well plates. The Auto Focus feature was used to set the focus. (B) An example image of well A1 using the Auto Focus setting.
Results
The CHO-S cells grown in CloneMedia CHO Growth A formed compact, spherical colonies that are easy to identify. The wells seeded at 500 cells/mL were at a density that enabled individual colonies to be viewed on Day 8 (Figure 4A and 4B). The use of semi-solid media allows a single well to grow multiple distinct colonies, which enables researchers to screen the same number of colonies using fewer plates and less media compared to limiting dilution.
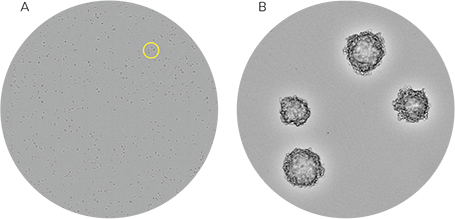
Figure 4: CHO-S cells in CloneMedia CHO Growth A media images captured from the CloneSelect Imager. (A) Whole-well image of CHO-S cells seeded at a density at 500 cells/mL. The CHO-S cells were imaged on Day 8 using the CloneSelect Imager. (B) A zoomed-in portion of the well shows distinct colonies. The yellow circle on (A) denotes the region of the well that is shown in (B).
By using the CloneSelect Imager software to zoom in on individual, immobilized colonies, it is possible to track growth and determine whether a colony is monoclonal by reviewing the colony images at different time points. In Figure 5, the images of two colonies are shown. By observing growth of the two colonies and tracking the images back to Day 0, it can be determined whether or not the colony originated from one or more cells. The addition of the beads (shown in the yellow circles) to the semi-solid media serves as a location reference to confirm that the same colony is being imaged each time.
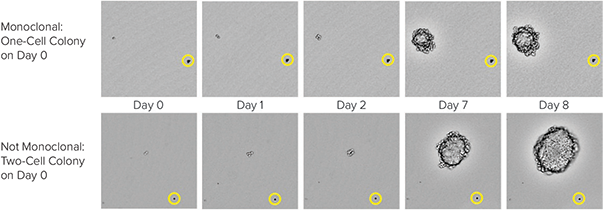
Figure 5: CHO-S cell growth in CloneMedia CHO Growth A semi-solid media. CloneSelect Imager was used to capture images from the 6-well plates at multiple time points. On Day 0, it is clearly observed on the top that one cell is present while on the bottom, two cells are observed. The yellow circles show the position of a bead that serves as a location reference to confirm that the same colony is imaged over time.
Conclusion
Growing CHO cells in CloneMedia CHO Growth A is a more efficient way to clone CHO cells than performing limiting dilution in liquid media. Semi-solid media immobilizes the cells which prevents the cells from moving during routine handling. This enables researchers to confidently track the growth of a single cell into a colony. While the CloneSelect Imager has traditionally been used to image cells in liquid media, it also has the ability to image cells grown in semi-solid media, thus providing a tool to monitor cell growth as well as to determine monoclonality.