
Application Note
Estimation of Protein Molecular Weights with ScanLater Western Blot Protein Ladder
- A single set of standards contains markers for both visual gel and blot alignment plus TRF detection.
- Proteins are biotinylated
- Molecular weights of detected proteins can be estimated
- No mixing or heating required
Introduction
The ScanLater™ Western Blot Protein Ladder is an essential component of the ScanLater™ Western Blot Detection System. The primary applications of this protein ladder include the estimation of protein molecular weight, the visualization of gel electrophoresis, and the evaluation of the transfer process from gel to blot. The ScanLater Western Blot Protein Ladder consists of seven biotinylated recombinant proteins as well as three prestained marker proteins. Detection of the biotinylated proteins is enabled by adding Europium-labeled streptavidin to the blot at the secondary antibody step. The blot is read with the ScanLater™ Western Blot Detection Cartridge on the SpectraMax® i3 or SpectraMax® Paradigm® Multi-Mode Microplate Readers.
ScanLater Western Blot Protein Ladder
The ScanLater Western Blot Protein Ladder consists of seven biotinylated recombinant proteins at 10, 20, 40, 50, 80, 100 and 140 kD. They are detected by incubating the blot with Europium-labeled streptavidin and they show as light colored bands when read with the ScanLater Western Blot Detection Cartridge (Figure 1, left). In addition, there are three visible pre-stained marker proteins at 18, 31, and 70 kD (Figure 1, right). These blue bands are seen on the electrophoresis gel and on the blot after transfer. They are also seen as dark bands when the ladder is read with the Scanlater Western Blot Detection System.
The linear relationship between the molecular weight of the biotinylated ladder proteins and their migration distance on the gel is examined in Figure 2 in comparison with a reference standard.
ScanLater Western Blot detection method
The ScanLater Western Blot workflow follows standard gel loading and blotting methods up to the secondary antibody incubation step. Along with protein samples of interest, simply load one of the lanes on the gel with 4 µL of ScanLater Western Blot Protein Ladder. No heating or other preparation of the ladder is required. After incubation with primary antibody, membranes undergo blocking and washing, followed by incubation with 1:5000 Eustreptavidin and 1:5000 Eu-secondary antibody. Eu-streptavidin binds to the protein ladder, and Eu-secondary antibody binds specifically to the primary antibody attached to the protein of interest.
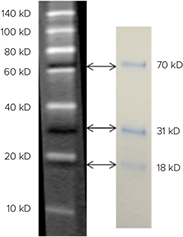
Figure 1: ScanLater Western Blot Protein Ladder. White bands are biotinylated protein used to estimate molecular weights of blotted proteins. They are detected after incubation with Eu-streptavidin and read on the ScanLater Western Blot Detection System. Dark bands are pre-stained protein used to monitor progress of protein electrophoresis, blot orientation, and assess transfer efficiency. They are visible as blue bands during these processes.
Membranes are scanned on either the SpectraMax Paradigm or SpectraMax i3 Multi-Mode Microplate Reader with the ScanLater Western Blot Detection Cartridge,which utilizes time resolved fluorescence (TRF) detection of Europium (Eu). The TRF detection significantly reduces background noise from auto fluorescence or other sources of short lifetime emissions. There is no camera blooming which is often seen with chemiluminescence or standard fluorescence detection; thus the system provides sharp bands with high S/N ratio. The method does not involve an enzymatic reaction, and as a result removes the inherent variability for protein quantitation. Europium is resistant to photo-bleaching, so the western blot signal remains stable for weeks to months which enables repeated reading of membranes. The ScanLater Western Blot Detection System, including the ScanLater Western Blot Protein Ladder, is a simple, sensitive, and stable platform that provides excellent protein analysis capability in a multi-mode microplate reader.
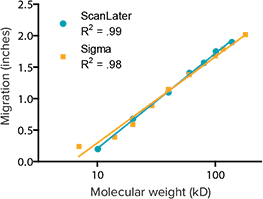
Figure 2: Migration vs. molecular weight. The migration distances of seven proteins in the ScanLater Western Blot Protein Ladder are plotted and fitted as a function of their respective molecular weights. The linear relationship is similar to that of a reference product from Sigma cat. #B2787.
Visual estimation of known proteins
Further experiments with Interleukin-17 and Heat Shock Protein also exemplify the use of the ScanLater Western Blot Protein Ladder for estimating molecular weight.
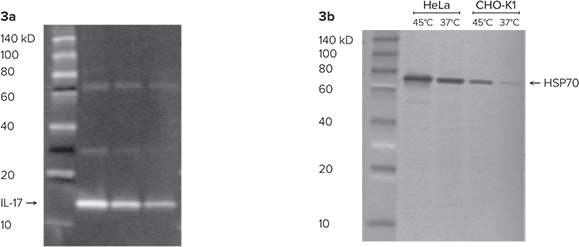
Figure 3: (3a) Molecular weight estimation of IL-17. Estimation of the molecular weight of IL-17 using the ScanLater Western Blot Protein Ladder. (3b) Western blot showing an increase in HSP70. Western blot shows an increase in HSP70 that occurs when cells are exposed to temperatures that are higher than normal. Estimate of molecular weight is approximately 70 kD.
Interleukin-17
Interleukin-17 (IL-17) was loaded on to a 4-20% gradient gel in 1X running buffer and run for 30 minutes. ScanLater Western Blot Protein Ladder was also loaded onto the same gel. Proteins were transferred to an Immobilon-FL membrane and probed with rat anti-IL-17 overnight followed by incubation for one hour with 1:5000 ScanLater Eu-anti-rat IgG and 1:5000 ScanLater Eu-anti-streptavidin antibody. As shown in Figure 3a, the blot was washed, dried and scanned using SpectraMax i3 Multi-Mode Microplate Reader with ScanLater Western Blot Detection Cartridge. The molecular weight of IL-17 was estimated at approximately 14 kD using the ScanLater Western Blot Protein Ladder, compared to the published molecular weight range of approximately 15 kD.
Heat shock protein
Heat Shock Protein (HSP70) is induced in cells exposed to higher than normal incubation temperatures. In this study, cell extracts were collected from CHO-K1 cells and HeLa cells six hours after the cells were exposed to 45°C for 45-90 minutes. 5 µL of extract from each cell sample was run on a 4-20% gel gradient. 4 µL of ScanLater Western Blot Protein Ladder was also loaded onto the gel. After transfer, blots were blocked, washed and probed overnight with mouse anti-HSP70 followed by a one-hour incubation with Eu-anti-mouse IgG. In this experiment, the ladder was incubated separately with anti Eu-streptavidin. In a reverse image seen in Figure 3b, pre-stained standards appear as pale bands because the stain quenches the TRF signal. Both cell lines show an increase in HSP70 at increased temperature. Estimation by comparison to the ScanLater Western Blot Protein Ladder shows that the protein HSP70 is approximately 70 kD in size.
Conclusion
ScanLater Western Blot Protein Ladder allows estimation of protein size seen as light bands in Figure 1 while providing visible indicators on the gel during electrophoresis and on the blot during and after transfer. With this system, users can follow the electrophoresis and blotting workflow that is optimized for their application. Incubation with a cocktail of Eu-streptavidin and Eu-secondary antibody enables low background detection in TRF mode of both the ladder and proteins of interest on SpectraMax i3 or the Paradigm Multi-Mode Microplate Reader. Images are generated in SoftMax® Pro Software, which includes integrated export to a custom Excel macro for quantitative analysis. With the addition of the ScanLater Western Blot Protein Ladder, the ScanLater Western Blot Detection System provides excellent sensitivity and protein analysis capability in a multi-mode microplate reader.
* Visit our website to see a complete listing of ScanLater Western Blot reagents and systems
Compatible with these Molecular Devices systems

SpectraMax i3 and i3x Multi-Mode Microplate Reader
SpectraMax iD5 Multi-Mode Microplate Reader

SpectraMax Paradigm Multi-Mode Microplate Reader