
Application Note
Phenolic compounds measurement in red wines
- Increased throughput compared to traditional cuvette-based methods
- Automated data analysis with SoftMax Pro Software
- PathCheck Sensor for normalized absorbance readings in microplate wells
Introduction
Measurement of tannin, iron-reactive phenolics, anthocyanin and polymeric pigment in red wine is an important part of quality control in the wine industry. Precise and reliable measurement of phenolic compounds in wine is critical for making decisions during fermentation, maceration, pressing and blending. Harbertson et al. developed a comprehensive red wine phenolics assay in 2003.1 Traditionally this assay is performed using a cuvettebased UV-vis spectrophotometer. It is a time-consuming and laborious process of reading individual samples in separate cuvettes, recording the results, and analyzing the data. Identifying the need for a rapid and cost-effective way of performing the assay, Heredia et al. adapted the assay for a microplate platform. This approach increases the throughput with respect to labor, time and sample volume. The assay widens the capacity to monitor the fermentations for extraction of phenolic compounds in an average-sized winery. Here we describe the use of Molecular Devices SpectraMax® Plus 384 Microplate Reader and SoftMax® Pro Software to efficiently collect and analyze the data for this assay.
Some of the unique features of the SpectraMax Plus 384 Microplate Reader are:
- Wavelength Range: 190–1000 nm in 1-nm increments
- Dual monochromator-based optics eliminate the need for filters (Figure 1)
- Read Speed: 96 wells: 9 Seconds 384 wells: 29 Seconds
- Temperature: 4°C above ambient to 45°C
- Cuvette port: Holds standard cuvettes and 12 x 75 mm test tubes
- OD range: 0–4 OD
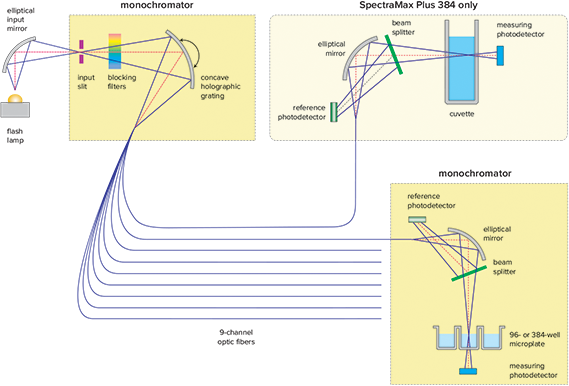
Figure 1. SpectraMax® Plus 384 optics.
Red wine phenolic assay
The Adams/Harbertson assay uses spectrophotometry, protein precipitation and bisulfite-bleaching techniques to measure red wine phenolics.
PathCheck Sensor
The PathCheck Sensor is a temperature independent feature from Molecular Devices that measures the optical pathlength of samples in microplate wells. It is an innovative way of normalizing the absorbance reading in a microplate well to that of a 1-cm cuvette..
Beer-Lambert Law states that
Absorbance = E * C * L
where
E = absorptivity (extinction coefficient)
C = concentration
L = pathlength
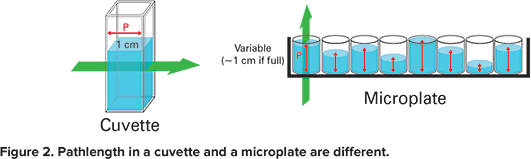
In the case of a cuvette, the optical path is horizontal. Hence the pathlength is fixed and is equal to 1 cm. But in the case of a microplate, the optical path is vertical. So, the pathlength depends on the volume of the sample (Figure 2). The PathCheck Sensor corrects for the discrepancy.
SoftMax Pro Software
The software controls the instrument, collects the data, and provides complete data analysis. Customized protocols with appropriate instrument settings and calculations can be pre-written and saved. The end user can conveniently open a preconfigured protocol and obtain complete results and analysis with no protocol setup time.
Materials
- Wine samples: Pinot noir (Sonoma Coast)
- Maleic acid (Fisher Scientific cat. #03417-500)
- Bovine serum albumin (Sigma cat. #A3803-10G)
- Triethanolamine (Sigma cat.#T1377-100 mL)
- Ferric chloride hexahydrate (Sigma cat.#236489-100G)
- (+)-Catechin (Sigma cat. #C1251-5G)
- Acetic acid (Sigma cat. #242853-2.5KG)
- Sodium chloride (Fisher Scientific cat. #S271-3)
- Sodium hydroxide (Fisher Scientific cat. #S318-500)
- Ethanol (Acros cat. #61509-0040)
- Potassium bitartrate (Sigma cat. #243531- 500G)
Instruments and accessories
- SpectraMax Plus 384 Microplate Reader (Molecular Devices cat. #PLUS 384)
- UV transparent 96-well microplates (Costar cat. #3635)
- Transferpette micropipette (Drummond Scientific cat. #2704174, 2705402, 2705412, 2704180; Drummond Digital Microdispenser cat. #3-000-510)
- Microtips for the micropipettes (Eppendorf cat. #epT.I.P.S.; Reloads cat. #022491539, 022491512, 022491547)
- Centrifuge for microfuge tubes (Eppendorf cat. #5424)
- Microfuge tubes 1.5 mL capacity (Eppendorf cat. #022364111, 022363557, 022363514, 2236357-3)
Buffer A
- 200 mM Acetic acid
- 170 mM NaCl
- Glacial acetic acid (6.0 mL)
- NaCl (4.97 g)
- 10% NaOH (adjust to pH 4.9)
Fill to 500 mL
DI H
2
O
Buffer B
- 12% EtOH (v/v
- 5 g/L Potassium bitartrate
- Potassium bitartrate (2.5 g)
- 200 proof ethanol (60 mL)
- 2.0 N HCl (adjust to pH 3.3)
Fill to 500 mL
DI
H2O
Buffer C
- 5% Triethanolamine (v/v)
- 5% SDS (w/v)
- Sodium docecyl sulfate (25 g)
- Triethanolamine (25 mL)
- 2.0 N HCl (adjust to pH 9.4)
Fill to 500 mL
DI
H2O
Buffer D
- 200 mM Maleic acid
- 170 nM NaCl
- Maleic acid (11.61 g)
- NaCl (4.97 g)
- 10% NaOH (adjust to pH 1.8)
Fill to 500 mL
DI
H2O
Ferric chloride
- 0.01 N HCl
- 10 mM FeCl 3
- Ferric chloride hexahydrate (0.27 g)
- 12.1 N HCl (80 µL)
Fill to 100 mL
DI
H2O
Bleaching
- 0.36 M K 2 S 2 O 5
- Potassium metabisulfite (0.395 g)
Fill to 5.0 mL
DI
H2O
Catechin
- 1 mg/mL (+)-Catechin
- 10% Ethanol (v/v)
- (+)-Catechin (50 mg)
- 200 Proof ethanol (5.0 mL)
Fill to 50 mL
DI
H2O
Protein
- 1 mg/mL BSA
- Bovine serum albumin (50 mg)
Fill to 50 mL
Buffer A
Table 1. Preparation of reagents for comprehensive red wine phenolics assay (summarized from Harbertson et al. 2003, Picciotto 2002).
Methods
Catechin standard curve
A set of dilutions was made to cover a range of 0–300 mg/L catechin in duplicate in a microplate. The final volume was adjusted to 262 µL with Buffer C. The plate was shaken on the shaker and 38 µL of ferric chloride solution was added to each well. The plate was shaken, further incubated for 10 minutes and the absorbance was read at 510 nm in the microplate reader. Buffer C was used as a blank.
Tannin analysis
Wine samples were diluted in Buffer B as per Heredia et al. 500 µL of diluted wine sample was added to a 1.5 mL microfuge tube. Next, 1 mL of buffer A with BSA (final concentration 1 mg/mL) was added to the tube and the reagents were mixed immediately by inverting the tube. The microfuge tube was centrifuged at 13,500 g to pellet the tannin-protein complex. Supernatant was removed from the microfuge tubes and the pellet was redissolved in 300 µL of Buffer C. A 262-µL aliquot was transferred to the microplate, and absorbance at 510 nm was read in the microplate reader with Buffer C as a blank (tannin background measurement). 38 µL of ferric chloride reagent was added to each of the wells, mixed using a multichannel pipetter, and incubated for 10 minutes at room temperature. A second reading at 510 nm was taken with Buffer C as a blank (tannin final measurement). The amount of tannin in each sample was calculated as described in Table 2 and expressed in mg/L catechin equivalents.
Component
a
x Dilution factors
b
Abs due tannin
c
= [(Tannin Final)–(zero catechin)]– (Tannin Background * 0.875)
- Tannin Final = 0.50, Tannin Background = 0.05, zero catechin = 0.002, intercept (b) = 0.0075, slope (m) = 0.0053;
- Abs due tannin = [0.50-0.002]–(0.05*0.875) = 0.4542
- Tannin = x = (y–b)/m then multiply by factors = [(0.4542–0.0075)/0.0053] *2*5 = 843 mg/L CE
Abs due IRP
d
= [(IRP Final)–(zero catechin)]–(IRP Background * 0.875)
- IRP Final = 0.80, IRP Background = 0.10, zero catechin = 0.002, intercept (b) = 0.0075, slope (m) = 0.0053;
- Abs due IRP = [0.80-0.002]–(0.10*0.875) = 0.7105
- IRP = x = (y–b)/m then multiply by dilution factor = [(0.7105-0.0075)/0.0053]*20 = 2,652 mg/L CE
D x 10
A x 5
- D = 0.4503, A = 0.3800;
- Anthocyanin = [(0.4503*10)–(0/3800*5)]/0.0102 = 255 mg/L M-3-G
x 3
x 1.08
x 4/3
- B = 0.2200, C = 0.1200;
- LPP = [(0.2200–0.1200)*3*1.08*4/3*5 = 2.16 AU
x 3
x 1.08
x 10/7
- C = 0.1200;
- SPP = 0.1200*3*1.08*10/7*5 = 2.78 AU
a Abbreviations: CE, catechin equivalent; IRP, Iron-reactive phenolics; M-3-G, malvidin-3-glucoside; LLP, large polymeric pigment; SPP, small polymeric pigment; AU, absorbance unti.\ b Dilution factor of 5 used fo rall wines except Pinot noir, for which a factor of 1 was used. Dilution factor of 20 used for all IRP calculations.\ c Insert “Abs due tannin” (y) into equation of line from catechin standard curve and solve for x, where y = mx+b and then Tannin = x = (y–b)/m.\ d Insert “Abs due IRP” (y) into equation of line from catechin standard curve and solve for x, where y = mx+b and then IRP = x = (y–b)/m.
Table 2. Summary of calculations for comprehensive red wine phenolics assay (from Harbertson et al. 2003, Picciotto 2002).
Iron reactive phenolics (IRP) analysis
15 µL of an undiluted wine sample was added to the microplate well followed by addition of 247 µL of buffer C. The reagents were mixed with the multichannel pipetter. The reaction was incubated at room temperature for 10 minutes, and an absorbance reading was taken at 510 nm with Buffer C as a blank (IRP background measurement). Following this, 38 µL of ferric chloride was then added to each well. The reagents were mixed with the multichannel pipetter. The reaction was incubated at room temperature for 10 minutes, and an absorbance reading was taken at 510 nm with Buffer C as a blank (IRP final measurement). The amount of IRP in each sample was calculated as described in Table 2 and expressed in mg/L catechin equivalents.
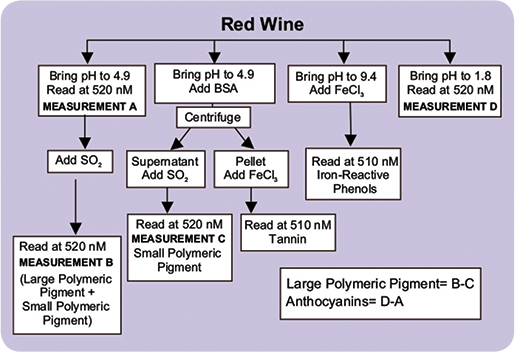
Figure 3. Red wine assay principle.
Anthocyanin analysis
Wine samples were diluted in Buffer B as per Heredia et al. 500 µL of a diluted wine sample was added to a 1.5 mL microfuge tube. Next, 1 mL of buffer A was added to the tube and the reagents were mixed immediately by inverting the tube. A 300 µL aliquot was transferred to the microplate well in duplicate and incubated for 10 minutes at room temperature. Measurement A was obtained at 520 nm with Buffer A as blank.
A volume of 50 µL of a diluted wine sample was added per well, followed by the addition of 50 µL of Buffer B. To this reaction mixture, 200 µL of Buffer D was added and mixed with multichannel pipetter. The reaction was incubated for 10 minutes at room temperature, followed by measurement of absorbance at 520 nm (measurement D). The amount of anthocyanin in each wine sample was calculated as described in Table 2 and expressed as mg/L malvidin-3-glucoside (M-3-G) units (Picciotto, E.A., et al.).
Instrument setup
The instrument was programmed through the SoftMax Pro Software. The settings were fed in the plate section of the software (Figure 4).
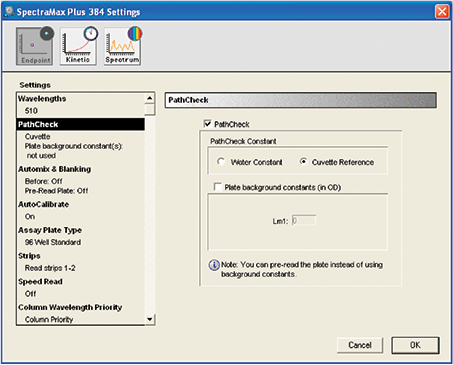
Figure 4. Instrument settings for catechin and tannin assay. Pathcheck Sensor settings are shown. Cuvette reference was selected if the reaction took place in a non-aqueous (organic) environment. For aqueous reactions, Water Constant was selected.
Step 1. The reading type was endpoint.
Step 2. The wavelength was set at 510 nm (for tannin, catechin and ironreactive phenolics) or at 520 nm (for the measurements A, B, C, D) by typing the appropriate number in the wavelength option.
Step 3. “PathCheck” option was selected. If the reaction took place in an aqueous environment, the option for Water Constant was selected, and the software used the constant included in the firmware. If the reaction took place in non-aqueous environment, the option for cuvette reference was selected. During this reading, a cuvette filled with the organic buffer was inserted in the cuvette port. The instrument took the reading from the cuvette and used it to correct the pathlength.
Step 4. Automixing was not selected.
Step 5. With autocalibration, the instrument calibrated itself for the selected wavelength.
Step 6. Appropriate plate format and wells to be read were selected.
Template setup
A template was set up in the software to denote placement of the series of concentrations in the appropriate wells. Flexible template layout allows easy addition of more samples and replicates.
Figure 5 is an example of a template setup for the catechin standard curve. Appropriate templates were set up for the rest of the analyses.
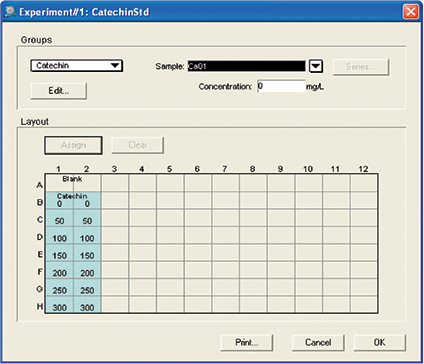
Figure 5. Template setup for catechin assay. Template setup for RS assay. For the initial plate, no template was set up. For the final plate blank, standards and unknown samples were assigned.

Figure 6. Reduction setup for catechin assay. Reduction setup showing custom formula being used for the subtracting optical density of each well in the initial plate from the optical density of the same well in the final plate. PathCheck Sensor values were applied for the calculation.
Results
Catechin standard curve
A standard curve for catechin (0–300 mg/mL) was prepared as per Materials and Methods. SoftMax Pro Software automatically subtracted the blank and calculated mean, standard deviation and %CV. The software also plotted a standard curve of OD at 510 nm against catechin concentration that was used for calculation of tannin and IRP concentrations in the wine sample by interpolation. Note the zero catechin concentration was pulled out below in a summary line below the group section (Figure 7).
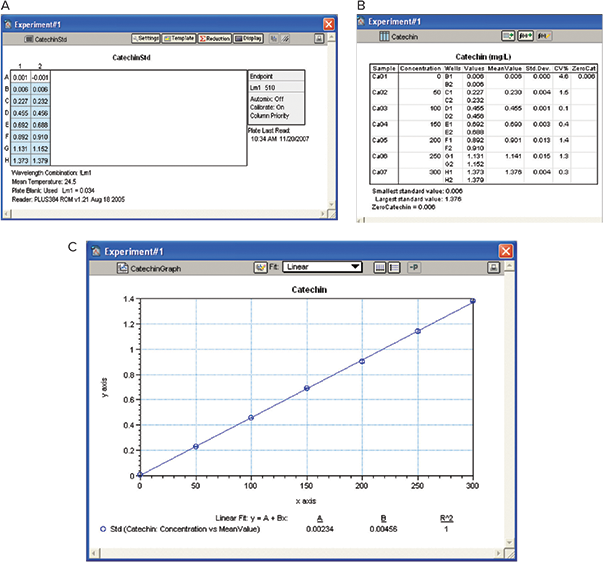
Figure 7. Catechin standard curve. A: Plate View. B: Results. Catechin standard curve was used for interpolation in calculating tannin and IRP concentrations.
Analysis of tannin
Analysis of tannin was carried out as described in Materials and Methods. The software was programmed to calculate the optical density due to tannin with the following formula:
Abs due to tannin = {(Tannin final)–(Zero catechin)}–(Tannin background * 0.875)
The software utilized the OD of the zero concentration of catechin from the summary line of the catechin group section. The tannin concentration was calculated by interpolation from the catechin standard curve after applying the correction factor to account for reaction dilution and dilution of wine. In this example, the dilution factor was not used, as the wines were not diluted (Figure 8).
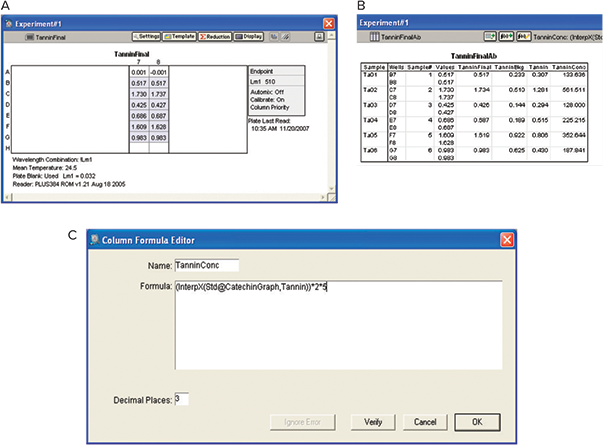
Figure 8. Analysis of tannin. A: Plate View. B: Results. OD values were interpolated using catechin standard curve to obtain tannin concentration with the custom formula as shown.
Analysis of Iron Reactive Phenolics (IRP)
Analysis of IRPs was carried out as described in the Materials and Methods section. The software was programmed to calculate the optical density due to IRP with the following formula:
Abs due to IRP = {(IRP final)–(Zero catechin)}–(IRP background * 0.875)
The software used the OD of the zero concentration of catechin from the summary line of the catechin group section. The IRP concentration was calculated by interpolation from the catechin standard curve after applying the correction factor to account for dilution of wine (Figure 9).

Figure 9. Iron Reactive Phenolics (IRP). A: Plate View. B: Results. OD values were interpolated using catechin standard curve to obtain IRP concentration with the custom formula as shown..
Analysis of anthocyanins
Analysis of anthocyanins was carried out as described in Materials and Methods. The software was programmed to calculate the concentration of anthocyanins with the following formula:
((Measurement D*10)–(Measurement A*5)) /0.0102
Here, numbers 10 and 5 represent the respective dilution factors, and 0.0102 is the conversion factor needed to express the anthocyanin concentration in malvidin- 3-glucoside (M-3-G) units (Picciotto, E.A. et al.) (Figure 10).
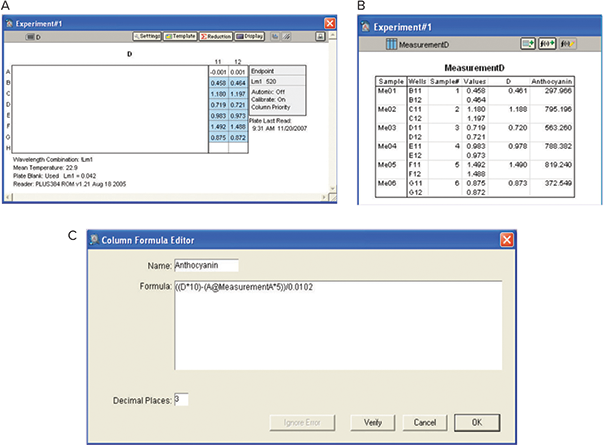
Figure 10. Analysis of anthocyanins. A: Plate View. B: Results. OD values were interpolated using catechin standard curve to obtain anthocyanins concentration with the custom formula as shown.
Conclusion
- The SpectraMax Plus 384 Microplate Reader is a good choice for running red wine phenolics assay described by Heredia et al. in a 96-well microplate.
- Other Molecular Devices readers with absorbance detection mode, e.g. the SpectraMax i3x Multi-Mode Microplate Reader, may also be used for this application.
- Using the microplate format is both costand time-effective.
- The tunability and PathCheck Sensor features of the SpectraMax Plus 384 Microplate Reader aid in achieving higher precision and accuracy.
- SoftMax Pro Software is a convenient tool for analysis and calculation of complicated and large data sets. It offers pre-written, ready-to-use protocols, custom formulas, and appropriate graphing options.
- For increased throughput requirements, Molecular Devices StakMax® Microplate Handling System integrates with SpectraMax readers and enables automated processing of batches of 20, 40, or 50 microplates.
References
- Harbertson J F et al.; (2002) Am J Enol Vitic 53: 54-59
- Harbertson J F et al.; (2003) Am J Enol Vitic 54: 301-306.
- Harbertson J F et al.; (2004) Am J Enol Vitic 55: 295A.
- Heredia T M et al.; (2006) Am J Enol Vitic 57: 497-502.
- Picciotto E A et al.; (2002) Thesis, University of California, Davis.
介绍
红酒中单宁,铁反应性酚类,花青素和聚 合物色素的测定是葡萄酒行业质量控制的 重要组成部分。准确、可靠地测量葡萄酒 中的酚类化合物对于发酵,浸渍,压榨和 混合过程中的决策至关重要。Harbertson 等人于 2003 年开发了一种全面的红酒酚类 测定法 (1)。传统上,该测定法是使用基于 比色皿的紫外可见分光光度计进行的。读 取单个比色皿中的单个样品,记录结果并 分析数据是一个耗时且费力的过程。 Heredia 等人认为需要一种快速、经济有 效的检测方法,因此将该检测方法应用于 微孔板平台。这种方法增加了有关人工, 时间和样品量的通量,扩大了中等规模酒 厂中监测酚类化合物提取发酵过程的能 力。在这里,我们描述了使用 Molecular Devices 公司的 SpectraMax®Plus 384 微 孔板读板机和 SoftMax®Pro 软件来有效收 集和分析此测定的数据。
SpectraMax Plus 384 微孔板读板机的一 些独特功能包括:
- 波长范围:190‒1000 nm,以 1 nm 为 增量
- 基于双单色器的光学元件使其无需使用 滤光片 ( 图 1 )
- 读取速度:96 孔:5 秒 384 孔:16 秒
- 温度:比环境温度高 4 °C 至 45 °C
- 比色皿插槽:可容纳标准比色皿和 12 x 75 mm 试管
- OD 范围:0‒4 OD

图 1 SpectraMax®Plus 384 光学器件
红酒酚含量测定
Adams / Harbertson 测定法使用分光光 度法、蛋白质沉淀法和亚硫酸氢盐漂白技 术来测量红酒中的酚类。
PathCheck传感器
PathCheck® 传感器是 Molecular Devices 的一项专利[1]功能,可以测量微孔板中样 品的光程。 这是将微孔板中的吸光度读数 标准化为 1 厘米比色皿的创新方法。
Beer-Lambert定律指出吸光度 = E * C * L
其中
E = 吸收率 ( 消光系数 )
C = 浓度
L = 光程

在比色皿的情况下,光路是水平的。因此, 光程是固定的,等于 1 cm。但是在微孔板 的情况下,光路是垂直的。因此,光程长 短取决于样品的体积 ( 图 2 )。 PathCheck 传感器会纠正差异。
SoftMax Pro 软件
该软件控制仪器,收集数据并提供完整的 数据分析。 带有适当的仪器设置和计算方 法的客制化模板可以预先编写并保存。 最 终用户可以方便地打开预先设置的模板, 获得完整的结果和分析,而无需重新花费 时间设置模板。
实验材料
- 葡萄酒样品: Pinot noir (Sonoma Coast)
- 马来酸 (Fisher Scientific cat. #03417-500)
- 牛血清白蛋白 (Sigma cat. #A3803-10G)
- 三乙醇胺 (Sigma cat.#T1377-100 mL)
- Ferric chloride hexahydrate (Sigma cat.#236489-100G)
- (+)- 儿茶素 (Sigma cat. #C1251-5G)
- 乙酸 (Sigma cat. #242853-2.5KG)
- 氯化钠 (Fisher Scientific cat. #S271-3)
- 氢氧化钠 (Fisher Scientific cat. #S318-500)
- 乙醇 (Acros cat. #61509-0040)
- 酒石酸氢钾 (Sigma cat. #243531- 500G)
仪器及配件
- SpectraMax Plus 384 微孔板读板机 (Molecular Devices cat. #PLUS 384)
- 紫外可透 96 孔微孔板 (Costar cat. #3635)
- 微量移液器 (Drummond Scientific cat. #2704174, 2705402, 2705412, 2704180; Drummond Digital Microdispenser cat. #3-000-510)
- 微量移液器的微量吸头 (Eppendorf cat. #epT.I.P.S.; Reloads cat. #022491539, 022491512, 022491547)
- 微量离心管离心机 (Eppendorf cat. #5424)
- 1.5 mL 微量离心管 (Eppendorf cat. #022364111, 022363557, 022363514, 2236357-3)
Buffer A
- 200 mM Acetic acid
- 170 mM NaCl
- Glacial acetic acid (6.0 mL)
- NaCl (4.97 g)
- 10% NaOH (adjust to pH 4.9)
Fill to 500 mL
DI H
2
O
Buffer B
- 12% EtOH (v/v
- 5 g/L Potassium bitartrate
- Potassium bitartrate (2.5 g)
- 200 proof ethanol (60 mL)
- 2.0 N HCl (adjust to pH 3.3)
Fill to 500 mL
DI
H2O
Buffer C
- 5% Triethanolamine (v/v)
- 5% SDS (w/v)
- Sodium docecyl sulfate (25 g)
- Triethanolamine (25 mL)
- 2.0 N HCl (adjust to pH 9.4)
Fill to 500 mL
DI
H2O
Buffer D
- 200 mM Maleic acid
- 170 nM NaCl
- Maleic acid (11.61 g)
- NaCl (4.97 g)
- 10% NaOH (adjust to pH 1.8)
Fill to 500 mL
DI
H2O
Ferric chloride
- 0.01 N HCl
- 10 mM FeCl 3
- Ferric chloride hexahydrate (0.27 g)
- 12.1 N HCl (80 µL)
Fill to 100 mL
DI
H2O
Bleaching
- 0.36 M K 2 S 2 O 5
- Potassium metabisulfite (0.395 g)
Fill to 5.0 mL
DI
H2O
Catechin
- 1 mg/mL (+)-Catechin
- 10% Ethanol (v/v)
- (+)-Catechin (50 mg)
- 200 Proof ethanol (5.0 mL)
Fill to 50 mL
DI
H2O
Protein
- 1 mg/mL BSA
- Bovine serum albumin (50 mg)
Fill to 50 mL
Buffer A
表 1 红酒酚类物质全面检测试剂的制备 ( 摘自 Harbertson 等人 2003,Picciotto 2002 )
实验方法
儿茶素标准曲线
在微孔板中进行一系列稀释,以包括 0-300 mg/L 儿茶素,每个浓度两个复孔。 用缓冲液 C 将最终体积调节至 262 µL。 将板在振荡器上振荡,并向每个孔中加入 38 µL 氯化铁溶液。将板进行震荡,进一 步孵育 10 分钟,并在酶标仪中于 510 nm 读取吸光度。 缓冲液 C 用作空白。
单宁分析
根据 Heredia 等人的方法,将葡萄酒样品在 缓冲液 B 中稀释。将 500 µL 稀释的葡萄酒 样品添加到 1.5 mL 微量离心管中。接着, 将 1 mL 具有 BSA ( 终浓度为 1 mg/mL ) 的 缓冲液 A 加入到离心管中,并且通过颠倒 离心管立即混合试剂。将微量离心管以 13,500 g 离心以沉淀单宁蛋白复合物。从微 量离心管中移出上清液,将沉淀重新溶解 在 300 µL 缓冲液 C 中。将 262 µL 等分试 样转移至微孔板,并在微孔板读取器中读 取 510 nm 的吸光度,缓冲液 C 为空白 ( 单 宁背景测量 )。将 38 µL 氯化铁试剂添加到 每个孔中,使用多通道移液器混合,并在 室温下孵育 10 分钟。用缓冲液 C 作为空 白在 510 nm 处进行第二次读数 ( 单宁最终 测定 )。 如表 2 中所述计算每个样品中单 宁的量,并以 mg/L 儿茶素当量表示。
Component
a
x Dilution factors
b
Abs due tannin
c
= [(Tannin Final)–(zero catechin)]– (Tannin Background * 0.875)
- Tannin Final = 0.50, Tannin Background = 0.05, zero catechin = 0.002, intercept (b) = 0.0075, slope (m) = 0.0053;
- Abs due tannin = [0.50-0.002]–(0.05*0.875) = 0.4542
- Tannin = x = (y–b)/m then multiply by factors = [(0.4542–0.0075)/0.0053] *2*5 = 843 mg/L CE
Abs due IRP
d
= [(IRP Final)–(zero catechin)]–(IRP Background * 0.875)
- IRP Final = 0.80, IRP Background = 0.10, zero catechin = 0.002, intercept (b) = 0.0075, slope (m) = 0.0053;
- Abs due IRP = [0.80-0.002]–(0.10*0.875) = 0.7105
- IRP = x = (y–b)/m then multiply by dilution factor = [(0.7105-0.0075)/0.0053]*20 = 2,652 mg/L CE
D x 10
A x 5
- D = 0.4503, A = 0.3800;
- Anthocyanin = [(0.4503*10)–(0/3800*5)]/0.0102 = 255 mg/L M-3-G
x 3
x 1.08
x 4/3
- B = 0.2200, C = 0.1200;
- LPP = [(0.2200–0.1200)*3*1.08*4/3*5 = 2.16 AU
x 3
x 1.08
x 10/7
- C = 0.1200;
- SPP = 0.1200*3*1.08*10/7*5 = 2.78 AU
a Abbreviations: CE, catechin equivalent; IRP, Iron-reactive phenolics; M-3-G, malvidin-3-glucoside; LLP, large polymeric pigment; SPP, small polymeric pigment; AU, absorbance unti.\ b Dilution factor of 5 used fo rall wines except Pinot noir, for which a factor of 1 was used. Dilution factor of 20 used for all IRP calculations.\ c Insert “Abs due tannin” (y) into equation of line from catechin standard curve and solve for x, where y = mx+b and then Tannin = x = (y–b)/m.\ d Insert “Abs due IRP” (y) into equation of line from catechin standard curve and solve for x, where y = mx+b and then IRP = x = (y–b)/m.
表 2 全面红酒酚类物质测定的计算总结 ( 摘自 Harbertson 等人,2003;Picciotto 2002 )
铁反应性酚类 (IRP) 分析
将 15 µL 的未稀释葡萄酒样品添加到微孔 板孔中,然后添加 247 µL 的缓冲液 C。将 试剂用多通道移液器混合。 将反应在室温 下温育 10 分钟,并用缓冲液 C 作为空白 在 510 nm 处读取吸光度 ( IRP 背景测量 )。 随后,将 38 µL 氯化铁添加到每个孔中。 将试剂用多通道移液器混合。将反应在室 温下温育 10 分钟,并使用缓冲液 C 作为 空白在 510 nm 处读取吸光度 ( IRP 最终测 量 )。如表 2 中所述计算每个样品中的 IRP 量,并以 mg/L 儿茶素当量表示。

图 3 红酒检测原理
花青素分析
根据 Heredia 等人的方法,将葡萄酒样品 在缓冲液 B 中稀释。将 500 µL 的稀释酒样 品添加到 1.5 mL 微量离心管中。 接下 来,将 1 mL 缓冲液 A 添加至离心管中, 并通过倒转离心管立即混合试剂。将 300 µL 等分试样一式两份转移至微孔板孔中, 并在室温下孵育 10 分钟。以缓冲液 A 为 空白在 520 nm 获得测量值 A。
每孔添加 50 µL 稀释的葡萄酒样品,然后 添加 50 µL 缓冲液 B。向该反应混合物中 添加 200 µL 缓冲液 D 并用多通道移液器 混合。将反应在室温下温育 10 分钟,然 后测量 520 nm 处的吸光度 ( 测量值 D )。 如表 2 中所述计算每个葡萄酒样品中的花 青素含量,并以 mg/L 氯化锦葵色素 3-葡 糖苷 (M-3-G) 单位表示 ( Picciotto,E.A 等 )。
仪器设置
仪器通过 SoftMax Pro 软件编程,设置在 软件的 plate 部分 ( 图 4 )。

图 4 儿茶素和单宁测定的仪器设置。 。PathCheck 传感器设置如图所示。 如果反应在非水 (有机) 环 境中进行,请选择比色杯参考。 对于水性反应,选择水常数
步骤 1. 读取类型为终点法。
步骤 2. 通过在波长选项中键入适当的数字, 将波长设置为 510 nm ( 单宁,儿茶素和铁 反应性酚类 ) 或 520 nm ( 测量值 A,B, C,D )。
步骤 3. 选择“ PathCheck”选项。如果反 应在水性环境中进行,则选择“水常数” 选项,则软件将使用固件中包含的常数。 如果反应在非水环境中进行,则选择比色 皿参考选项。在读取过程中,将装有有机 缓冲液的比色皿插入比色皿槽中。仪器从 比色皿中读取读数,并用它来校正光程。
步骤 4. 不选择自动混匀。
步骤 5. 通过自动校准,仪器会针对所选波 长进行自我校准。
步骤 6. 选择适当的平板格式和要读取的孔。
模板设置
在软件中设置一个模板,以指示一系列浓 度在适当孔中的位置。灵活的模板布局可 轻松添加更多样品和重复孔。图 5 是儿茶 素标准曲线的模板设置示例。为其他的分 析设置了适当的模板。
Figure 5 is an example of a template setup for the catechin standard curve. Appropriate templates were set up for the rest of the analyses.

图 5 儿茶素测定的模板设置。 RS 分析的模板设置。对于初始板,未设置模板。对于最终的板,分 配了空白,标准品和未知样品组

图 6 儿茶素测定的运算设置。图 6 儿茶素测定的运算设置。 运算设置显示自定义公式,该公式用于从最终板中同一孔的光密度 中减去初始板中每个孔的光密度。将 PathCheck®Sensor 值应用于计算
实验结果
儿茶素标准曲线
c根 据 实 验 材 料 和 实 验 方 法 制 备 儿 茶 素 (0‒300 mg/mL) 的标准曲线。SoftMax Pro 软件自动减去空白并计算出平均值,标准 偏差和 % CV。该软件还绘制了 510 nm OD 对儿茶素浓度的标准曲线,该曲线用于 通过插入计算葡萄酒样品中的单宁和 IRP 浓度。请注意,零儿茶素浓度在分组部分 下方的摘要行中被列出 ( 图 7 )。

图 7 儿茶素的标准曲线。 A:平板视图。B:结果。采用儿茶素标准曲线插入法计算单宁和IRP浓度
单宁分析
如实验材料和实验方法中所述进行单宁的分析。通过以下公式对软件进行编程以计算单宁引起的光密度:
Abs due to tannin = {(Tannin final)–(Zero catechin)}–(Tannin background * 0.875)
该软件利用了儿茶素分组部分摘要行中的 儿茶素浓度为零的 OD。在应用校正因子考 虑反应稀释度和葡萄酒稀释度后,通过儿 茶素标准曲线的插入计算单宁浓度。在此 示例中,未使用稀释因子,因为葡萄酒未 稀释 ( 图 8 )。

图 8 丹宁的分析。 。A:平板视图。B:结果。利用儿茶素标准曲线插值 OD 值,得到单宁浓度,自定 义公式如图所示
铁反应性酚类(IRP)分析
如实验材料和实验方法中所述进行IRP的 分析。通过以下公式对软件进行编程以计 算 IRP 引起的光密度:
Abs due to IRP = {(IRP final)–(Zero catechin)}–(IRP background * 0.875)
该软件使用了儿茶素分组部分摘要行中儿 茶素浓度为零的 OD。应用校正因子考虑 葡萄酒的稀释度后,通过儿茶素标准曲线 的插入来计算 IRP 浓度 ( 图 9 )。

图 9 铁反应性酚类 (IRP)。 Plate View. B: A:平板视图。B:结果。利用儿茶素标准曲线插值 OD 值,得到 IRP 浓度, 自定义公式如图所示
花青素分析
如实验材料和实验方法中所述进行花青素的分析。通过以下公式对软件进行编程以计算花青素的浓度:
((Measurement D*10)–(Measurement A*5)) /0.0102
此处,数字 10 和 5 代表各自的稀释因子, 而 0.0102 是表示以氯化锦葵色素 3-葡糖 苷 (M-3-G) 单位表示花青素浓度所需的转 化因子 ( Picciotto,E.A 等 ) ( 图 10 )。

图 10 花青素的分析。: A:平板视图。B:结果。利用儿茶素标准曲线插值 OD 值,得到花青素浓度, 自定义公式如图所示
结论
- SpectraMax Plus 384 微孔板读板机是在 96 孔板中进行 Heredia 等人描述的红酒 酚类测定的理想选择。
- 其他具有吸光度检测模式的 Molecular Devices 微孔板读板机,例如 SpectraMax i3x 多模式微孔板读板机也可用于此应 用。
- 使 用 微 孔 板 形 式 既 节 省 成 本 又 节 约 时 间。
- SpectraMax Plus 384 微孔板读板机的可 调性和 PathCheck 传感器功能有助于实 现更高的精度和准确性。
- SoftMax Pro 软件是用于分析和计算复 杂和大型数据集的便捷工具。它提供了 预先编写的,随时可用的模板,自定义 公式以及适当的图形选项。
- 为了提高通量要求,Molecular Devices StakMax® 微孔板处理系统与 SpectraMax 读板机集成在一起,可以自动处理20、 40 或 50 个微孔板。
References
- Harbertson J F et al.; (2002) Am J Enol Vitic 53: 54-59
- Harbertson J F et al.; (2003) Am J Enol Vitic 54: 301-306.
- Harbertson J F et al.; (2004) Am J Enol Vitic 55: 295A.
- Heredia T M et al.; (2006) Am J Enol Vitic 57: 497-502.
- Picciotto E A et al.; (2002) Thesis, University of California, Davis.