
Lectores de placas de absorbancia
Lectores de microplacas y espectrofotómetros para detección de absorbancia visible y UV-visible
Espectrofotómetros SpectraMax para detección de absorbancia UV-Vis
Los lectores de placas y espectrofotómetros de absorbancia SpectraMax® proporcionan versatilidad y comodidad para una amplia variedad de ensayos como ELISA, cuantificación de ácidos nucleicos y proteínas así como proliferación microbiana.
Nuestros lectores de placas de absorbancia cuentan con nuestra tecnología PathCheck Sensor y el software de adquisición y análisis de datos SoftMax® Pro líder del mercado. Pueden combinarse con nuestro apilador de microplacas StakMax® y se pueden integrar fácilmente con los sistemas robóticos de socios líderes.
También proporcionamos herramientas de validación y cumplimiento BPx para el rendimiento óptimo de laboratorios BPF/BPL.
Nuestros destacados lectores de microplacas SpectraMax ABS/ABS Plus
Los lectores de microplacas SpectraMax ABS/ABS Plus proporcionan detección rápida de absorbancia sin el uso de filtros con selección de longitud de onda con monocromador para absorbancia visible y UV-visible.

ÓPTICA CON OCHO CANALES
La lectura de la placa se realiza en tan solo cinco segundos

MICROPLACAS O CUBETAS
Utilice una microplaca de 96 o 384 pocillos o una cubeta estándar

TECNOLOGÍA PATHCHECK SENSOR
Mide la trayectoria óptica de las muestras

Software SoftMax Pro
Herramienta de adquisición y análisis de imágenes líder del mercado

Validación y cumplimiento BPx
Mediciones verificadas con el software y las placas de validación SpectraTest

Automatización robótica
Se integra con los apiladores y sistemas de automatización de socios
Aplicaciones y ensayos de absorbancia
Con más de 40 años de experiencia en lectores de placas e investigación en ciencias de la vida, hemos acumulado una amplia colección de contenido centrado en aplicaciones en nuestro Centro de recursos. Nuestras notas de aplicación de absorbancia destacadas incluyen:
- Viabilidad celular, citotoxicidad, proliferación celular
- -Ensayos MTT
- -Ensayos XTT
- -Ensayos MTS
- Cuantificación de ADN/ARN
- Cinética enzimática, proliferación microbiana/bacteriana
- Ensayos de endotoxinas
- ELISA/inmunoensayos
- -Cuantificación de antígenos, anticuerpos, citoquinas, etc.
- Aplicaciones de alimentos y bebidas
- Aplicaciones con microvolúmenes
- Cuantificación de proteínas
- -Ensayos BCA
- -Ensayos Bradford
- -Ensayos Lowry
Comparación de producto de lectores de absorbancia de modo único
wavelength-ranges
microplate-types
reading-speed
cuvette-port
photometric-accuracy
shaking
¿Cuál es la diferencia entre un espectrómetro y un lector de microplacas de absorbancia?
Un espectrómetro estándar mide la absorbancia de una sola muestra en un momento determinado. La muestra normalmente se pone en una cubeta a través de la cual hace pasar la luz horizontalmente. Un lector de placas de absorbancia ofrece un mayor rendimiento y puede medir la absorbancia de las muestras en microplacas (normalmente de 96 pocillos o incluso de 384 pocillos) haciendo pasar la luz a través de cada pocillo verticalmente.
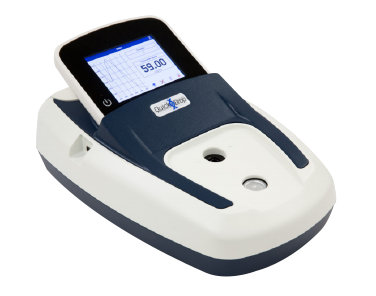
Espectrofotómetro SpectraMax QuickDrop Micro-Volume
Cuantificación exacta de ADN, ARN y proteínas con un solo toque en un lector de absorbancia de microvolumen de espectro completo.
Recursos destacados de absorbancia
APLICACIÓN
Ensayo de inmunoadsorción ligado a enzima (ELISA)
El ELISA (ensayo de inmunoadsorción ligado a enzima) es un método utilizado para detectar cuantitativamente un antígeno en una muestra. Un antígeno es una toxina o cualquier otra sustancia extraña, por ejemplo, el virus de la gripe o un contaminante ambiental, que provoca que el sistema inmunitario de los vertebrados inicie una respuesta defensiva.

TECNOLOGÍA
Absorbancia
La absorbancia (A), también conocida como densidad óptica (DO), es la cantidad de luz absorbida por una solución. La transmitancia es la cantidad de luz que pasa a través de una solución.

SEMINARIO WEB
Explorando las aplicaciones de ensayos basados en la absorbancia: Desde la investigación de virus a la de cannabis
La absorbancia, también conocida como densidad óptica, es la cantidad de luz absorbida por una solución. Los lectores de microplacas con función de lectura de absorbancia se utilizan ampliamente en investigación biológica y química, en el descubrimiento de fármacos y en control de calidad.

eBook sobre ensayos de absorbancia
Optimice los ensayos de absorbancia para la cuantificación de proteínas y ácidos nucleicos
Los lectores de microplacas de absorbancia se utilizan ampliamente en investigación básica, descubrimiento de fármacos, validación de bioensayos, control de calidad y procesos de fabricación en las industrias farmacéutica, biotecnológica, de alimentos y bebidas y en el sector académico.

EBOOK SOBRE ALIMENTOS Y BEBIDAS
Optimice los análisis de seguridad y el control de calidad de cerveza, vino y alimentos
Obtenga nueva información con las aplicaciones para el pequeño pero potente lector de microplacas de absorbancia.
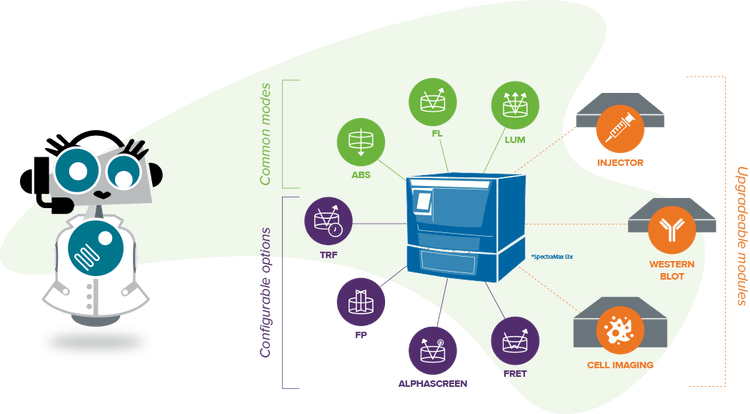
Lectores de microplacas multimodo con detección de absorbancia

Productos y servicios relacionados
Solución completa de herramientas y servicios, así como una amplia variedad de consumibles y ensayos para todas las necesidades del espectrofotómetro de su laboratorio.

Software
Software de control y análisis de datos del lector de microplacas con protocolos preconfigurados y flujo de trabajo de ensayos personalizado.

Kits de ensayo
Kits de ensayo sólidos y fáciles de usar para investigación en ciencias biológicas, descubrimiento y desarrollo de fármacos y bioensayos.
Consumibles
Variedad de material de laboratorio de alto rendimiento que incluye cartuchos, módulos, filtros y microplacas, por nombrar algunos.

Servicios
Personalice sus instrumentos, así como los flujos de trabajo completos automáticos para cubrir las necesidades específicas de su ensayo, método o protocolo.
Soluciones de cumplimiento BPx
Garantice el cumplimiento continuo y esté preparado para auditorías con nuestros servicios de validación y cumplimiento IQ/OQ/PM.

Lector de placas multimodo
Detección de absorbancia, fluorescencia y luminiscencia con funciones de alto rendimiento actualizables.
